

Indian Journal of Science and Technology
Year: 2021, Volume: 14, Issue: 2, Pages: 113-118
Original Article
Manoj R Gaware1*
1Department of Chemistry, M. V. P. Samaj’s K.P.G. Arts, Science and Commerce College, Igatpuri, Nashik, 422 403, Maharashtra, India. Tel.: +9193702935304
*Corresponding Author
Tel: +9193702935304
Email: [email protected]
Received Date:24 November 2021, Accepted Date:05 January 2021, Published Date:15 January 2021
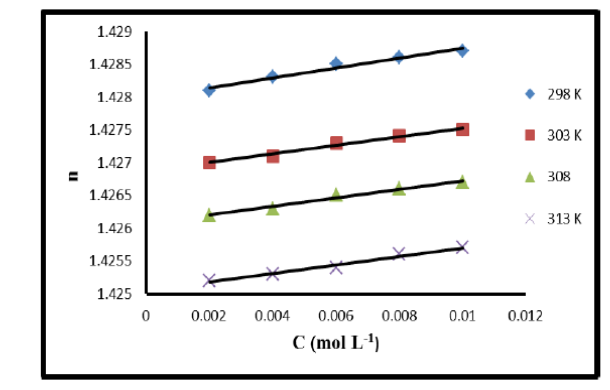
Objectives: The main purpose of this study is to investigate the impact of 6-(4-chlorophenyl)-1, 2, 3, 4-tetrahydro-2, 4-dioxopyrimidine-5-carbonitrile in binary solution at different concentrations and valuable information on electronic polarizability and molecular interactions in the solution. Methods: 6-(4-chlorophenyl)-1, 2, 3, 4-tetrahydro-2, 4-dioxopyrimidine-5-carbonitrile was synthesized and purified by recrystallization technique in laboratory(1). DMSO used is of analytical reagent grade (AR) of minimum assay of 99.9%. Stock solutions of 0.02 (mol L-1) of compound were prepared by dissolving calculated amounts in 60% dimethyl sulphoxide solution. Solutions in various concentration ranges from 0.002 to 0.010(mol L-1). The pycknometer was calibrated by measuring the densities of triple distilled water. The densities of distilled organic liquids like acetone, toluene and carbon tetrachloride were evaluated with respect to density of water. Refractive index were measured by Abbe’s Refractometer (AR-56, COSLAB) having range 1.3000 to 1.7100. The desired temperature was maintained by circulating water through refractometer. Findings: In the present work we found that density, refractive index, molar refraction and polarizability constant increases the increase in concentration due to decrease in angle of refraction or increase in angle of incidence. The decrease in density with increase in temperature is due to increase in molar volume of solvent. However, the decrease in refractive index is due to the fact that the solute-solute and solute-solvent interactions weaken with increase in temperature. This can be correlated that change in these properties changes the intermolecular attraction between solute and solvent. With increase in temperature the interaction between solute and solvent weakens and hence molar refraction and polarizability decreases. Novelty: The work incorporated with the study of molar refraction and molar polarizability of 6-(4-chlorophenyl)-1, 2, 3, 4-tetrahydro-2, 4-dioxopyrimidine-5-carbonitrilehas been not studied until now. So we wish to present the systematic measurement of refractive index of 6-(4-chlorophenyl)-1, 2, 3, 4-tetrahydro-2, 4-dioxopyrimidine-5-carbonitrile in 60% DMSO in the temperature range 298 to 313 K at different concentration. From refractive index we have calculated molar refraction and molar polarizability which has provided important information regarding interactions between solute and solvent.
Keywords: 6-(4-chlorophenyl)-1; 2; 3; 4-tetrahydro-2; 4-dioxopyrimidine-5-carbonitrile; refractive index; molar refraction; molar
polarizability
© 2021 Gaware.This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Published By Indian Society for Education and Environment (iSee)
Subscribe now for latest articles and news.