

Indian Journal of Science and Technology
Year: 2021, Volume: 14, Issue: 28, Pages: 2337-2341
Original Article
Ketan Soni1*, Kavita Sharma2
1Research Scholar, Shri Vaishnav Vidyapeeth Vishwavidyalaya, Indore, Tel.: 9827039131
2Professor & Co-ordinator, Shri Vaishnav Institute of Forensic Science, SVVV, Indore
*Corresponding Author
Tel: 9827039131
Email: [email protected]
Received Date:19 March 2021, Accepted Date:08 July 2021, Published Date:20 August 2021
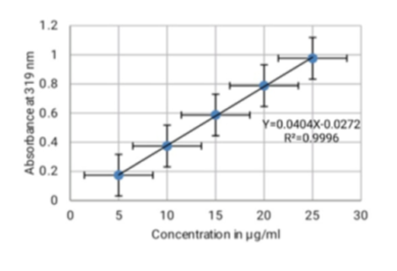
Objectives: To investigate an eco-friendly method to enhance the solubility of Mefenamic acid. The present investigation was to employ these hydrotropic solutions to extract the drugs from their dosage forms, precluding the use of costlier and harmful organic solvents. Methodology: Mefenamic acid was analyzed by using UV Visible spectrophotometer (Model 1800, Shimadzu), and its solubility (poorly water-soluble drug) measured by mixed solvency method. Sodium caprylate solution was used as a hydrotropic solubilizing agent. Findings: The solubility of the Mefenamic acid drug in water was very low at about 0.2 mg/ml and the solubility of Mefenamic acid in the 20% sodium caprylate solution was 10 mg/ml. The value of percentage estimation obtained was from 98.6 (tablet II) to 98.8 (tablet I). This value is obtained near to 100% hence, we can say that the proposed method is correct. Standard deviation (0.173 to 0.346), percentage coefficient of variation (0.175 to 0.350) and the value of standard error (0.101 to 0.202) are also very low to validate the accuracy of the proposed method. Novelty: Mixed solvency concept can be utilized for spectrophotometric estimation of poorly water-soluble drugs from their bulk drug samples to avoid the use of organic solvents that provide a new, economical, environmentally friendly, safe, and reliable analytical mechanism.
Keywords: Mixed solvency; solubility; hydrotropic; mefenamic acid; sodium caprylate
© 2021 Soni & Sharma. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Published By Indian Society for Education and Environment (iSee)
Subscribe now for latest articles and news.