

Indian Journal of Science and Technology
DOI: 10.17485/IJST/v15i47.1975
Year: 2022, Volume: 15, Issue: 47, Pages: 2673-2679
Original Article
Mahendra B Dhande1*, D T Tayade2
1Assistant Professor, Department of Chemistry, HPT Arts & RYK Science College, Nashik, 422005, India
2Professor, Department of Chemistry, Government Vidarbha Institute of Science and Humanities, Amravati, India
*Corresponding Author
Email: [email protected]
Received Date:03 October 2022, Accepted Date:28 November 2022, Published Date:24 December 2022
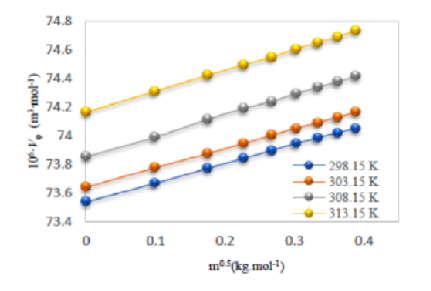
Objectives: To produce new and accurate data of density of aqueous solution of amino acid salts at different temperatures and to correlate it with molality. Methods: The densities of aqueous solutions of sodium salts of Amino acids such as L-cysteine, L-leucine, L-proline and L-valine have been measured using double-arm Pycnometer, placed in a transparent glass-walled water bath having thermal stability of (0.01 K) at concentrations range (0.01 to 0.15) mol L-1 and at 298.15, 303.15, 308.15, and 313.15 K. From density data ahead, apparent molar volumes (Vϕ ), the partial molar volumes (Vϕ 0), expansion coefficient (E¥) and Hepler’s constant ( ¶ 2Vϕ 0=¶ T2) were calculated and analysed on the basis of the intermolecular interaction and molecular structure. Findings: The densities are observed to increase with concentration and decrease with increasing temperature. The positive values of partial molar volume (Vϕ 0) indicate strong sodium amino acids -water interactions. Novelty: For studied aqueous sodium salt solution of Amino acids, such properties have not yet been reported in the open literature and at such a lower concentrations range (0.01 to 0.15) mol L-1.
Keywords: Density; Sodium Salt Amino Acid; Apparent Molar Volume; Helper’s Constant
© 2022 Dhande & Tayade. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Published By Indian Society for Education and Environment (iSee)
Subscribe now for latest articles and news.