

Indian Journal of Science and Technology
DOI: 10.17485/IJST/v17i11.3275
Year: 2024, Volume: 17, Issue: 11, Pages: 979-989
Original Article
Fatima A Al-Qadri1*, Raiedhah Alsaiari2, Mervate Mohamed Mohamed3, Esraa Mohamed3, Faeza Alkorbi3, Norah Alsaiari3, Moustafa A Rizk2
1Professor of Empty Quarter research Unit, Department of Chemistry, College of science and art in Sharurah, Najran University, Kingdom of Saudi Arabia
2Associate Professor of Empty Quarter research Unit, Department of Chemistry, College of science and art in Sharurah, Najran University, Kingdom of Saudi Arabia
3Assistant professor of Empty Quarter research Unit, Department of Chemistry, College of science and art in Sharurah, Najran University, Kingdom of Saudi Arabia
*Corresponding Author
Email: [email protected]
Received Date:31 December 2023, Accepted Date:04 February 2024, Published Date:29 February 2024
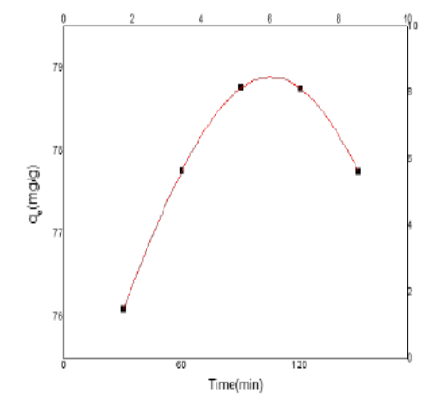
Objectives: This study describes the creation of a low-cost silica material using a silicate extract as a precursor. The main objective of this work is to develop an inexpensive sample to use as green removal for toxic heavy metals from water sources. Methods: This work produces Silica ash from combustion palm frond as waste ash through a simple calcination process at 500°C and a green extraction with water. Nitrogen adsorption-desorption of the ash has done, FTIR analyses, and transmission electron microscopy were used to characterize the samples. Adsorption Cr(VI) equilibrium data onto silica ash was done by Batch process using 0.25 g of waste palm frond ash silica and 100 mL of solutions with varying beginning Cr(VI) concentrations ranging from 100 to 400 mg/L at 70 °C, normal pH, and continuous stirring for 24 hours at a constant agitation speed. The effect of different conditions pH, temperatures, initial concentrations, dosage, and time on the adsorption capacity were investigated. The adsorption isotherms were fitted using Langmuir and Freundlich models, the adsorption kinetics were evaluated using pseudo-first order and pseudo-second order models also thermodynamic parameters were calculated. Findings: The extracted silica FTIR spectra were analyzed with main characteristic peaks at 500–1590 and 490–510 cm were assigned to (Si-O-Si), (Si-OH). N2 adsorption isotherm of the functionalized silica gave a hysteresis loop in the range of p/p° between (0.5 and 1). The resulting mesoporous silica ash material had a surface area of 226.36 m2/g and pore sizes of 6.7 nm. This low-cost silica ash-based adsorbent has a maximum Chromium Cr(VI) adsorption capacity of (83.79 mg/g) values. The removal of Cr(VI) was between 97% and 98 % by was for all used factors, the Adsorption obtained capacity q max (mg/g) by Langmuir was 58.87 mg/g and the n value obtained by Freundlich isotherm was 1.1. The negative results of thermodynamic parameters for adsorption of Chromium (VI) onto silica surface values suggested that the adsorption process was spontaneous and that the adsorbate's state at the solid/solution interface became less random and the values obtained were ∆S (-151.6 J.mol-1), ∆H° (-18.88 J.mol-1), and ∆G°(- 1797 J.mol-1). Novelty: The results show that produced silica from palm waste ash has a strong capability for eliminating toxic metals as Chromium (VI) as a low-cost alternative to commercial adsorbent.
Keywords: Palm frond, Silica, Chromium (VI), Adsorption, Kinetic, Thermodynamic, Waste ash
© 2024 Al-Qadri et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Published By Indian Society for Education and Environment (iSee)
Subscribe now for latest articles and news.