

Indian Journal of Science and Technology
DOI: 10.17485/IJST/v15i33.2336
Year: 2022, Volume: 15, Issue: 33, Pages: 1624-1633
Original Article
Nicha Thepsri1, Jasadee Kaewsrichan1*
1Department of Pharmaceutical Chemistry and Drug Delivery System Excellence Center, Faculty of Pharmaceutical Sciences, Prince of Songkla University, Hat-yai, Songkhla, 90112, Thailand
*Corresponding author
Email: [email protected]
Received Date:14 December 2021, Accepted Date:03 August 2022, Published Date:27 August 2022
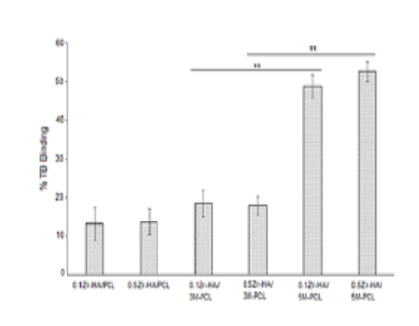
Objectives: To develop alkaline-treated polycaprolactone (PCL) and Zrhydroxyapatite (Zr-HA) and fabricate their mixtures as drug delivery systems for dental application. Methods: PCL was subjected to hydrolysis by using concentrated NaOH. Zr-HA was synthesized by co-precipitation reaction that contained Ca(NO3)2, (NH4)2HPO4 and ZrO2 at 65OC. The obtained materials were characterized by using techniques such as X-Ray Fluorescence, Fourier Transform Infrared Spectroscopy, X-Ray Diffraction, or rheometric analysis. Various compositions of the Zr-HA and the modified-PCL were used for preparing drug delivery systems, and properties including cytotoxicity, degradation, and toluidine blue (TB) binding/releasing were investigated. Findings: The synthesized Zr-HA was more non-stoichiometric than HAcontrol. Improved hydrophilicity was evident for the modified-PCL. Drug carriers composing of the Zr-HA and the modified-PCL were increasingly degradable in phosphate buffer saline solution compared to those containing HA/PCL-control, resulting in 9% and 6% weight loss after 8 weeks of the immersion, respectively. Binding of TB on the Zr-HA/the modified-PCL mixture increased while the release of such bound dye decreased in comparison with that of HA/PCL counterpart. All of the developed materials were non-cytotoxic based on MTT assay using L929 cell line. Novelty: Partial inclusion of Zr4+ ions in Ca2+ lattices of HA resulted in fairly degradable Zr-HA. Shortening the epsiloncaprolactone units of PCL by strong bases was simple in producing fragmented PCL polymer that exhibited improvement of hydrophilic interaction towards another. Varying the weight ratios of the Zr-HA and the modified-PCL when to prepare drug carriers is possible to acquire optimal binding/releasing profiles of drugs in dental cavities.
Keywords: Dental ceramic; hydroxyapatite; polycaprolactone; surface hydrophilicity; drug delivery system
© 2022 Thepsri & Kaewsrichan. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Published By Indian Society for Education and Environment (iSee)
Subscribe now for latest articles and news.