

Indian Journal of Science and Technology
Year: 2023, Volume: 16, Issue: 1, Pages: 66-76
Original Article
Treesa Varghese1,2*, Saleena Mathew2
1School of Biosciences, Mar Athanasios College for Advanced Studies, Tiruvalla, Kerala, India
2School of Industrial Fisheries, Cochin University of Science and Technology, Kochi (Retd), Kerala, India
*Corresponding Author
Email: [email protected]
Received Date:09 August 2022, Accepted Date:28 November 2022, Published Date:06 January 2023
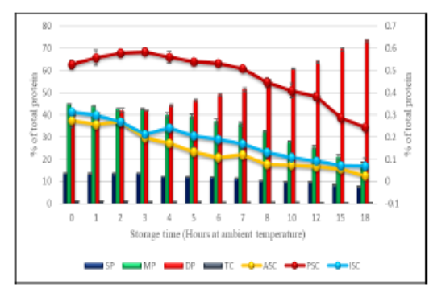
Objectives: To evaluate the physico-chemical and functional characteristics of the stinging catfish muscle tissue during the storage time of 18 hours following the death of the fish. Methods: In the present study, stinging catfish were collected from the paddy fields and streams near Tiruvalla Kerala. Fish were slaughtered immediately and analyzed for changes in pH value, cook loss, expressible water content, water holding capacity, sensory demerit score values, and textural profile and were compared with progress of rigor mortis. Study also evaluated the changes in protein fractions present in the muscle tissue and correlated them with autolytic enzyme activities. Findings: In stinging catfish, the rigor mortis occurred within four hours of storage (78.37 %) at ambient temperature. The post rigor stage was attained after the 6th hour and the K value reached above 60% within the 8th hour which indicates the unacceptable range for consumption. The present study also proved that the stinging catfish is an inosine producer. The initial concentration of sarcoplasmic protein in fish muscle (13.71%) decreased to 7.62% at the end of 18 hours of storage. The muscle tissue myofibrillar protein also showed a decrease from an initial concentration of 44.87% to a final concentration of 18.02%. Protein denaturation was evident with an increase from 39.65% to 73.20% at the end of the storage period. Collagen concentration was found to be reduced (31.85% reduction) after the 18th hour of storage. Pepsin soluble collagen was less susceptible to the activity of collagenase enzyme in the pre-rigor stage, confirming its role in post-rigor muscle softening when compared to acid soluble and insoluble collagen. Novelty: The present work authenticated that post-rigor softening of stinging catfish muscle is typically due to the degradation of pepsin soluble collagen and not reported elsewhere. Additionally, myofibrillar proteins have more influence on total hardness and stiffness than collagen, while cohesiveness is a better function of collagen than myofibrillar protein. These findings make it useful to perceive the process thatinduces softening in catfish and to create effective strategies to prevent it.
Keywords: Collagen; Myofibrillar Protein; Adenosine Nucleotides; Autolytic Enzymes; Textural Profile
© 2023 Varghese & Mathew. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Published By Indian Society for Education and Environment (iSee)
Subscribe now for latest articles and news.