

Indian Journal of Science and Technology
DOI: 10.17485/IJST/v17i25.2170
Year: 2024, Volume: 17, Issue: 25, Pages: 2570-2576
Original Article
Sachin Tripathi1∗, Seema Srivastava1, Vishal Singh Chandel2,3, Ameer Azam4, Upendra Kumar Mishra5,3
1Department of Physics, Integral University, Lucknow, 226026, Uttar Pradesh, India
2Department of Applied Science and Humanities, Rajkiya Engineering College, Ambedkar Nagar, 224122, Uttar Pradesh, India
3Dr. A.P.J. Abdul Kalam Technical university, Lucknow, 226031, Uttar Pradesh, India
4Department of Physics, Faculty of science, Islamic University of Madinah Al Jamiah, Madinah, 42351, Saudi Arabia
5Department of Applied Science, Shri Ramswaroop Memorial College of Engineering and Management, Lucknow, 226028, Uttar Pradesh, India
*Corresponding Author
Email: [email protected]
Received Date:25 August 2024, Accepted Date:05 June 2024, Published Date:18 June 2024
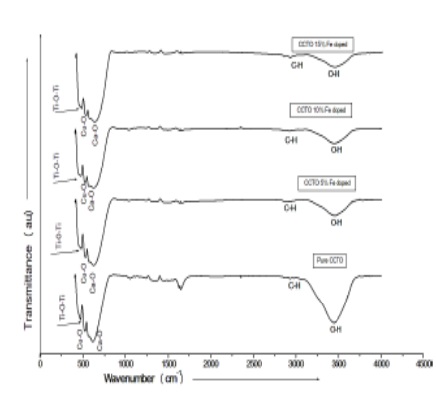
Objectives : The analysis of present material is to reveal the effect of Fe atom by replacing the Ti atom in CCTO for chemical properties. Methods: Pure and Fe doped CaCu3Ti4-xFexO12 (x=0.05, 0.10, and 0.15) CCTO ceramics were synthesized by solid-state reaction route and calcination was done at 930°C for 12 hrs with heating rate of 4°C/min. The phase structure of samples was confirmed by X-ray Diffraction (XRD) and found single phase calcium copper titanate. Morphology of all samples was investigated by scanning electron microscopy. Absorption band have been also recorded for all samples. Findings: The structure remains cubic by doping of Fe atom in place of Ti atom. The average size of all the samples were lies between 1-1.3µm. Novelty: The bond strength becomes stronger as the doping of Fe atom takes place. The large absorption band is found in the range 380-700 cm-1. The peak of large band is shifted towards lower wave number. These higher wavelengths can be used in water pollutants. The increased volume size of samples shows lower energy band gap. This lower energy band gap enhanced the electrical properties.
Keywords: FESEM, XRD, FTIR, EDS, CCTO
© 2024 Tripathi et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Published By Indian Society for Education and Environment (iSee)
Subscribe now for latest articles and news.