

Indian Journal of Science and Technology
Year: 2020, Volume: 13, Issue: 22, Pages: 2245-2263
Review Article
Mee Kin Chai1∗, Yeong Hwang Tan1 , Ling Shing Wong2
1 College of Engineering, University Tenaga Nasional, Jalan Ikram-Uniten, Kajang, 43000, Selangor, Malaysia. Tel.: +603-89287275
2 Faculty of Health and Life Science, INTI International University, Persiaran Perdana BBN, Putra Nilai, Nilai, 71800, Negeri Sembilan, Malaysia
∗Corresponding author:
Mee Kin Chai
College of Engineering, University Tenaga Nasional, Jalan Ikram-Uniten, Kajang, 43000, Selangor, Malaysia.
Tel: +603-89287275
Email: [email protected]
Received Date:07 April 2020, Accepted Date:09 June 2020, Published Date:28 June 2020
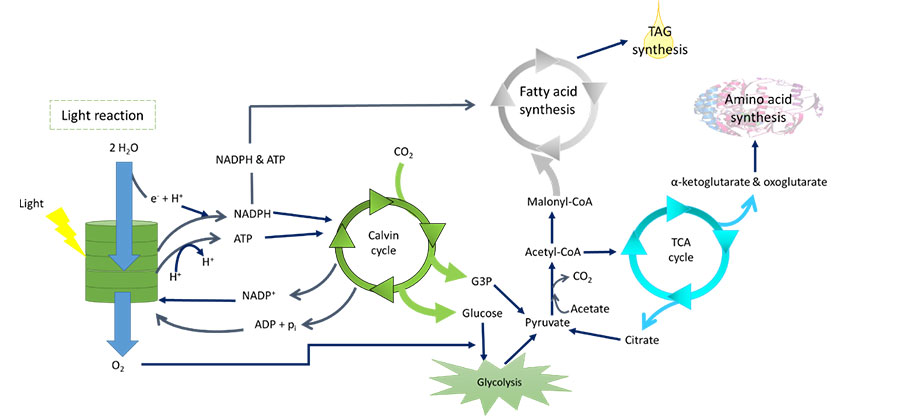
Objectives: This review is focused on the effect of macronutrients (nitrogen, carbon and phosphorus) on biomass production of microalgae especially concerned with biofuel. Methodology: The keyword search included "microalgae cultivation", "nitrogen sources", "phosphorus sources", "organic carbon", "biodiesel", "biofuel", "carbon dioxide", "inorganic carbon", "macronutrient deprivation", "macronutrient limitation", "lipid" and "organic waste" to search the published journals in ScienceDirect, Scopus, Springer, and Google Scholar. The search was performed from December 2019 until Mac 2020 to collect all the journals and books that are published between 2006 and 2020. The effect of each macronutrient (nitrogen, carbon and phosphorus) on microalgal growth of the control and the samples were compared using biomass productivity, concentration and biochemical content in each published article. Findings: Review shows that nitrogen has more pernicious effect than other macronutrients on most microalgal growth and lipid production. The concentrations and types of macronutrients have remarkable effects on the growth of microalgae; hence these criteria must be chosen scrupulously to achieve the desired biomass and metabolite production. In order to improve the biomass and biochemical productivity in concomitant with the cost reduction, replacement of cheap organic waste, genetic engineering of microalgae and two-stage hybrid system have been suggested to simultaneously maximize the biomass and biochemical production. The future research should focus on other biochemical contents such as carbohydrates, proteins and pigment to achieve the biorefinery context which can increase the profit. Besides, economic factor such as factorial design should be included in the future research to obtain the best combined factors with the maximum profit and minimal cost.
Keywords: Microalgae; biomass; macronutrient; biofuel
© 2020 Chai, Tan, Wong. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Published By Indian Society for Education and Environment (iSee)
Subscribe now for latest articles and news.