

Indian Journal of Science and Technology
Year: 2020, Volume: 13, Issue: 16, Pages: 1656-1667
Original Article
Rajeshri J Patel1∗, Lakshmi Bhaskaran1
1 Department of Microbiology, Shri M. M. Patel Institute of Science and Research, KSV, Gandhinagar, 382023, Gujarat, India. Tel.: +91-9904435363
∗Corresponding author:
Rajeshri J Patel
Department of Microbiology, Shri M. M. Patel Institute of Science and Research, KSV, Gandhinagar, 382023, Gujarat, India.
Tel: +91-9904435363
Email: [email protected]
Received Date:16 April 2020, Accepted Date:07 May 2020, Published Date:09 June 2020
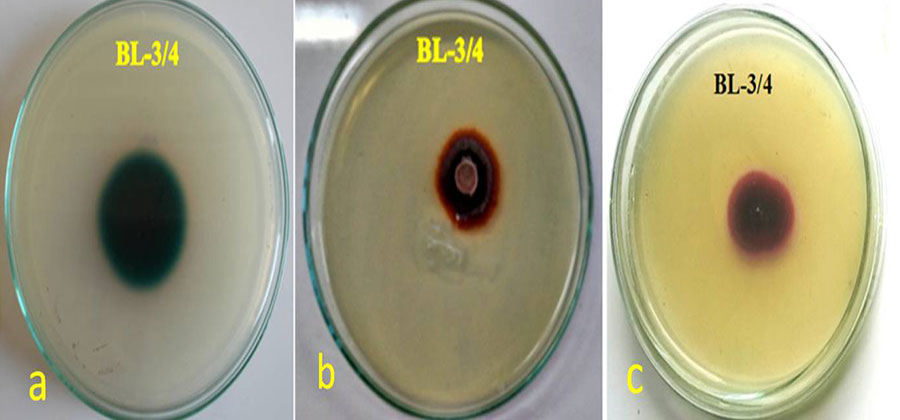
Objectives: Laccases are one of the ligninolytic enzymes with wide industrial applications hence objective of present study is to optimize laccase production in novel fungal strain Peyronellaea pinodella BL-3/4. Methodology: Fungal strains capable of oxidizing different lignin model compounds such as guaicol, syringaldazine and 2, 2'-Azino-bis (3ethylbenzthiozoline-6-sulphonic acid) were further tested for the laccase production in liquid media. 18s rRNA gene sequencing was performed to identify isolated novel fungal strain. Extracellular laccase activity from isolated fungal strain was optimized by the conventional `single parameter at a time' approach. Parameters used for this study included inoculum size, temperature, pH, agitation rate, lignocellulosic substrate, carbon source and nitrogen source. Findings: Among ten isolated laccase positive fungal strains, BL-3/4, exhibited maximum activity and morphological resemblance to Peyronellaea. 18s rRNA gene sequencing and phylogenetic analysis revealed that the isolated fungal strain is a novel one and identified as Peyronellaea pinodella BL-3/4. Optimization by single parameter approach leads to an 18 fold increase in laccase production by Peyronellaea pinodella BL-3/4. During the optimization of agro residues, orange peel acting as a substrate dramatically changed laccase production from 10.4 to 65.1 U/mL. Novelty: No reports are available on Peyronellaea pinodella laccase activity and optimization of various factors affecting the laccase production. Orange peelings (an agro waste)as a substrate has increased the laccase production by six-fold in P. pinodella, this makes the fungi a better candidate for large scale production of laccase as well as for bioremediation, when compared to all other reported fungi.
Keywords: Ascomycetes; Optimization; Aromatic inducers; Laccase activity; Lignin model compounds
© 2020 Patel, Bhaskaran. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Published By Indian Society for Education and Environment (iSee)
Subscribe now for latest articles and news.