

Indian Journal of Science and Technology
Year: 2020, Volume: 13, Issue: 15, Pages: 1589-1595
Original Article
Vikaskumar Shukla1∗, Jigar Patel1 , Laxman Bhutadiya1 , Jabali Vora2 , Jayesh Jadav3
1 Department of Chemistry, Sheth M N Science College, Patan, 384265, India
2 Department of Chemistry, Hemchandracharya North Gujarat University, Patan, 384265, India
3 Department of Chemistry, Samarpan Science and Commerce College, Ghandhinagar, 382028, India
∗Corresponding author
Vikaskumar Shukla
Department of Chemistry, Sheth M N Science College, Patan, 384265, India Email: [email protected]
Received Date:05 April 2020, Accepted Date:05 May 2020, Published Date:07 June 2020
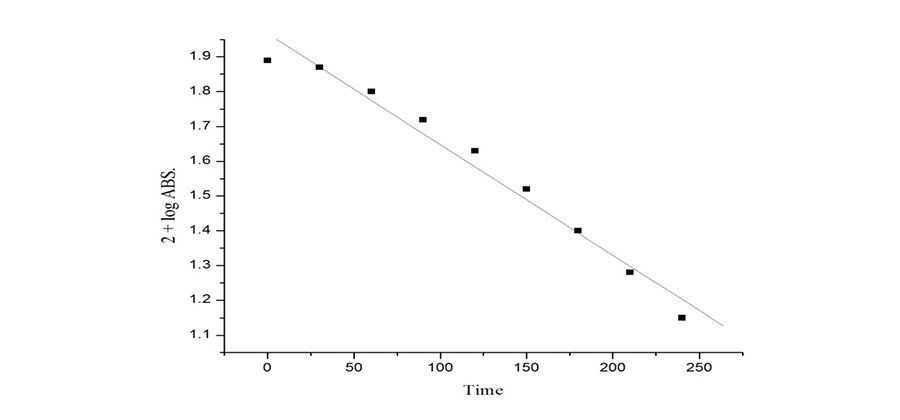
Objectives: This study was undertaken to examine the decolorization of mercuric picrate with ZnO as a photocatalyst. Methodology: The photo catalytic decolorization of mercuric picrate in the presence of heterogeneous semiconductor in the aqueous solution has been investigated. The progress of the reaction was checked by Shimadzu 1600 UV visible spectrophotometer at different time intervals. The effect of various operational parameters was studied such as the effect of mercuric picrate concentration, amount of photo catalyst, effect of light intensity, effect of band gap and effect of pH on the solution of mercuric picrate. The study on the effect of radial quenchers such as methanol and ethanol were used. Findings: The optimum conditions were obtained as pH =5.0, [Hg-picrate] = 6 ×10-5 M, amount of ZnO = 250 mg, light intensity =7.61 mWcm-2. The rate constent ontained was k= 7.30 × 10-3 min-1. The reaction proceeded through oxidation by hrdroxyl radial confirmed by scavenger. Zinc oxide is effective photocatalyst for decolorization of Hg-picrate. Other semiconductors like ZnS, CdS as well as PbS are not capable to carry out photoreaction. A tentative mechanism for this reaction has been proposed. Novelty: Mercuric Picrate is a probably explosive toxic substance. Hence a safe way to decompose this molecule into smaller and nonexplosive ways is of crucial importance. Photocatalysis is well established method. However, decomposition of mercuric picrate to a safe level can be achieved by this method.
Keywords: Advanced oxidation processes; decolorization; Zinc oxide; mercuric picrate
© 2020 Shukla, Patel, Bhutadiya, Vora, Jadav. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Published By Indian Society for Education and Environment (iSee)
Subscribe now for latest articles and news.