

Indian Journal of Science and Technology
DOI: 10.17485/IJST/v14i28.1407
Year: 2021, Volume: 14, Issue: 28, Pages: 2327-2336
Original Article
Pratima Rani Sardar1*
1Department of Botany, Sivaji College, University of Delhi, New Delhi, 110027
*Corresponding Author
Email: [email protected]
Received Date:02 August 2021, Accepted Date:09 August 2021, Published Date:18 August 2021
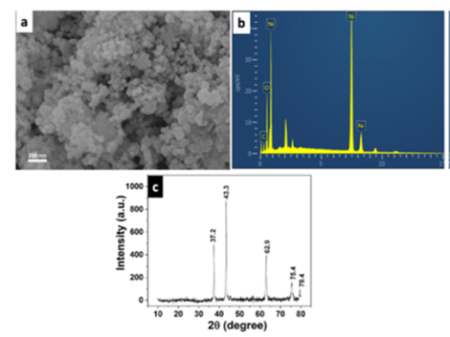
Objectives: To study the simultaneous removal of metal ions to understand the dynamics of the adsorption process for the assessment of the actual potential of an adsorbent in real-life applications. Methods: A one-step hydrothermal method was employed for the synthesis of nanomaterial. The hydrothermal treatment was performed at 110o C for 3 hours and calcined at 300o C for 2 hr to complete the nickel oxide nanoparticle synthesis. A systematic study of metal ion adsorption onto the nickel oxide nanoparticle was conducted to evaluate the maximum adsorption capacity and to understand the adsorption behaviour of the metal ions in presence of the others. The estimation of metal ion adsorption was done by measuring the residual concentrations using an atomic absorption spectrophotometer. Findings: The microscopic and spectroscopic characterizations confirmed the formation of nickel oxide nanostructures. The experimental results suggested that the adsorption process follows the Langmuir isotherm and the pseudo-secondorder model for metal ion adsorption in single and mixed solutions. A synergistic effect was observed for Pb (II) adsorption and an antagonistic effect for Cd (II) adsorption. The maximum adsorption capacity of ~650 mg/g of Pb (II) and ~475 mg/g of Cd (II) were noticed for simultaneous adsorption by the NiO nanoparticle. Novelty/improvement: The presence of more than one heavy metal ion in the wastewater is obvious, and one kind of metal ion may interface with the adsorption behaviour of the others. Further, limited studies on simultaneous adsorption of metal ions using metal-oxide nanoparticles are available in the literature. Hence, this work will provide an idea about the applicability of the NiO nanoparticle for real-life applications.
Keywords: Adsorption; NiO; Heavy metal ions; Simultaneous removal
© 2021 Sardar. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Published By Indian Society for Education and Environment (iSee)
Subscribe now for latest articles and news.