

Indian Journal of Science and Technology
Year: 2024, Volume: 17, Issue: 17, Pages: 1735-1744
Original Article
Bandari Vaishnavi1, G Tarun Reddy2, Akash Bansal2, Deva Likitha2, Deeksha Charagondla2, D Prasanthi3*
1M.Pharm. Student, Department of Pharmaceutics, G. Pulla Reddy College of Pharmacy, Mehdipatnam, Hyderabad, 500028, Telangana, India
2B.Pharm. Student, Department of Pharmaceutics, G. Pulla Reddy College of Pharmacy, Mehdipatnam, Hyderabad, 500028, Telangana, India
3Professor, Department of Pharmaceutics, G. Pulla Reddy College of Pharmacy, Mehdipatnam, Hyderabad, 500028, Telangana, India
*Corresponding Author
Email: [email protected]
Received Date:07 February 2024, Accepted Date:02 April 2024, Published Date:19 April 2024
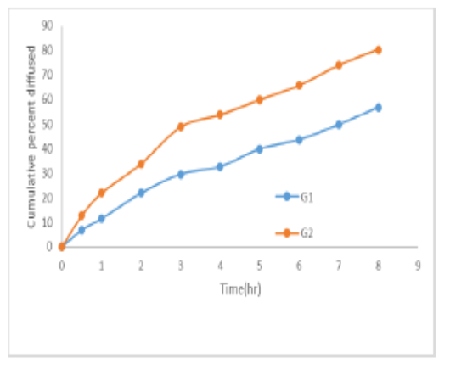
Objectives: Efinaconazole is an anti-fungal drug of BCS class II used for treating Onchomycosis. Neem gum known for its antifungal properties was purified and used as hydrophilic carrier. Solid dispersions by physical mixture and solvent evaporation method were prepared using purified Neem gum. Methods: The prepared solid dispersions in ratios 1:1, 1:3 and 1:6 were characterized for percentage yield, assay, solubility, and dissolution studies. The optimized solid dispersion SD6 was formulated as gel for ease of application. Gels were characterized for physico-chemical properties and in-vitro diffusion studies. The optimized gel was evaluated for anti-fungal activity. Findings: FTIR, DSC and XRD studies of optimized SD6 showed compatibility and amorphous nature respectively. G2 gel formulation containing SD6 solid dispersion showed 80.4±0.14% when compared to G1 56.76±0.34% release in 8hrs. Both the gels followed zero order and Higuchi release. Antifungal studies indicated more efficiency of G2 gel by zone of inhibition when compared with G1 containing only drug. G2 gel was found to be stable for one month. Novelty : In the present research work purified neem gum, which is natural carrier and neem known for its antifungal activity was used to enhance the solubility of drug and evaluate the antifungal activity of drug with purified neem gum. Conclusion: Hence it can be concluded, purified Neem gum can be used as hydrophilic carrier and as solubility of Efinaconazole was enhanced its antifungal activity was also increased.
Keywords: Onchomycosis, Efinaconazole, Neem gum, Solid dispersions, Antifungal
© 2024 Vaishnavi et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Published By Indian Society for Education and Environment (iSee)
Subscribe now for latest articles and news.