

Indian Journal of Science and Technology
DOI: 10.17485/IJST/v16i11.2364
Year: 2023, Volume: 16, Issue: 11, Pages: 803-815
Original Article
S Anbalagan1,2, M Ravishankar1*, S Sivakumar3, M Sivakumar3, E Krishna4
1Department of Chemistry, Rajah Serfoji Government College, Affiliated to Bharathidhasan University, Thanjur, Tamil Nadu, India
2Department of Chemistry, Laxminarayana College of Arts & Science (women), Dharmapuri, Tamil Nadu, India
3Department of Chemistry, E. R. K Arts and Science College, Dharmapuri, Tamil Nadu, India
4Department of Chemistry, P.S.A Arts and Science College, Dharmapuri, Tamil Nadu, India
*Corresponding Author
Email: [email protected]
Received Date:08 December 2022, Accepted Date:27 January 2023, Published Date:15 March 2023
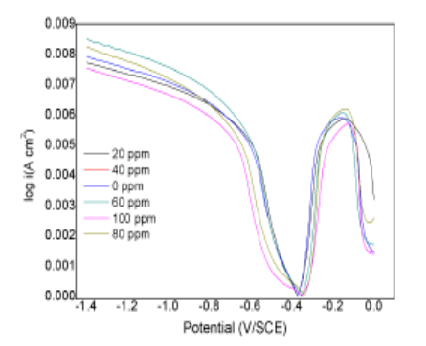
Objectives: To use the newly synthesized efficient organic corrosion inhibitor N-(4-((4-(pyridin-2-yl) piperazin-1-yl)methyl)phenyl)quinoline-6-carboxamide to carry out on mild steel corrosion inhibition study. Methods: N-(4-((4-(pyridin- 2-yl)piperazin-1-yl)methyl)phenyl)quinoline-6-carboxamide was synthesized by stirring method under 0oC to room temperature at 12 hrs. The synthesized organic inhibitor was characterized by 1H NMR, 13C NMR, UV and FTIR spectroscopy. The inhibition effect of organic inhibitor on the mild steel corrosion was investigated with weight loss measurement by gold measuring weighing balance. The inhibitor anchored down on the mild steel surface was analyzed by scanning electron microscopy and Atomic Force Microscopy. Findings: The inhibitor shows a better inhibition efficiency of maximum 74.41% in 1N HCl medium. In addition to this adsorption isothermal models were also interpreted to fit the adsorption behavior of the inhibitor compound on mild steel surface. Thus, the result shows the Langmuir adsorption isotherm. As a result, the interactions between inhibitor and mild steel surface have chemisorptions. Novelty: The N-(4-((4-(pyridin-2-yl) piperazin-1-yl)methyl)phenyl)quinoline-6-carboxamide shows better corrosion inhibition efficiency (74.41%). In addition, inhibitor and mild steel surface show chemisorptions interaction, which confirms free energy and enthalpy of adsorption.
Keywords: Carbaxamide Derivative; Scanning Electronic Microscopy; Atomic Force Microscopy Weight Loss Measurement And NMR
© 2023 Anbalagan et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Published By Indian Society for Education and Environment (iSee)
Subscribe now for latest articles and news.