

Indian Journal of Science and Technology
Year: 2021, Volume: 14, Issue: 15, Pages: 1219-1232
Original Article
S Dina1*, V T Thankamani2, T Emilia Abraham3
1Scientific Research Solution Private Limited, Karnataka, India. Tel.: 9845693891
2Professor and Head (Retd), Department of Biotechnology, University of Kerala,
Thiruvananthapuram, India
3Scientist G (Retd), National Institute for Interdisciplinary Science and Technology (CSIR),
Thiruvanathapuram, 695019, India
*Corresponding Author
Tel: 9845693891
Email: [email protected]
Received Date:09 February 2021, Accepted Date:21 March 2021, Published Date:03 May 2021
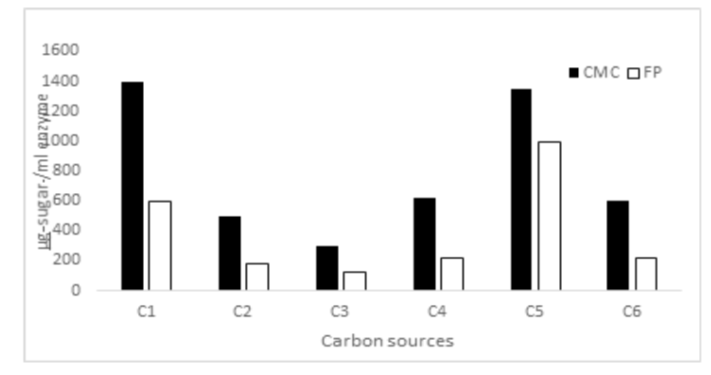
Background/Objectives: The importance of enzymatic bioconversion of cellulose cannot be understated and enzyme systems which complement existing enzymes, with broad ranges of pH and temperatures and hydrolyze cellulose synergistically with other enzymes are required for bioconversions. The study was undertaken to isolate and characterize cellulolytic organisms. Methodology: Isolation of cellulolytic strains from soil samples with degrading cellulosic wastes, screening and species level identification of Fungal strain was conducted. Stability of the enzyme systems over different pH and temperature and purification study of enzymes were carried out. Results: Fungal isolate, which exhibited 0.08 IU of filter paper (FP) activity, 0.33 IU of Carboxy methyl cellulose (CMC) activity and 15IU of xylanase activity was selected and identified as Aspergillus flavipes. Purification by ion exchange chromatography and gel filtration chromatography yielded a typical endoglucanase, a fraction showing activity with both avicel and caboxy mthyl cellulase(CMC) and a third fraction against CMC and avicel. GPC of third fraction showed that the enzymes had cross reactivity to the substrates used. Conclusion: Our study proved that Aspergillus flavipes is capable of degrading lignocellulose and its FP activity and CMC activity was higher than other isolates and was comparable to enzymes of a standard strain T. koninjii. To our knowledge this study is the first detailed report of a system of cellulases, suitable for saccharification of lignocellulosic biomass alone or synergistically with other enzymes produced by Aspergillus flavipes.
Keywords: Filter paper activity; CMC; glucosidase; endoglucanase; pNPG; lignocellulosic b saccharification
© 2021 Dina et al.This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Published By Indian Society for Education and Environment (iSee)
Subscribe now for latest articles and news.