

Indian Journal of Science and Technology
Year: 2020, Volume: 13, Issue: 26, Pages: 2678-2685
Original Article
A O Numbere1*
1Department of Animal and Environmental Biology, University of Port Harcourt, Choba Nigeria, P.M.B. 5323, Tel.: +2348056002989
*Corresponding author
Tel.: +2348056002989
Email: [email protected]
Received Date:31 May 2020, Accepted Date:22 June 2020, Published Date:30 July 2020
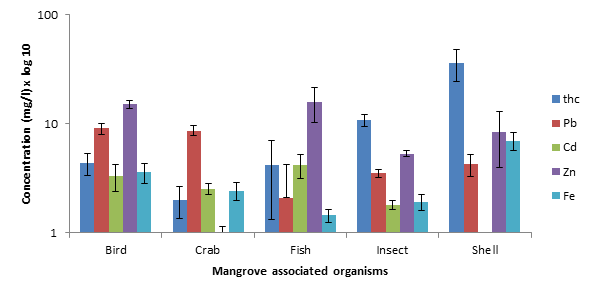
Background /Objectives: This study's objectives are (1) to determine the total hydrocarbon (THC) and heavy metals in sediment and water samples collected from mangrove forest at different locations; (2) to compare the THC and heavy metals across different mangrove associated species; (3) to compare the relationship between THC and heavy metals in different organisms across different locations and (4) to determine the THC and heavy metal concentration between vertebrates and invertebrate organisms in mangrove forest. Methods/ Statistical analysis: It is hypothesized that chemical contamination will bioaccumulate across multiple mangrove associated organisms. Physicochemical analysis for Cadmium (Cd), Zinc (Zn), Lead (Pb), Iron (Fe) and total hydrocarbon (THC), was carried out on sediment, water, crabs, fish, insect, anadara and bird droppings, and was measured by spectrophotometric method using HACH DR 890 colorimeter and microwave accelerated reaction system (MARS Xpress, North Carolina). Findings: There was a significant difference in chemical composition between mangrove associated species (F4, 145 = 2.83, P=0.03). Anadara has the highest THC (36.44±9/4mg/kg) and Iron (6.97±1.32mg/kg) concentrations while bird droppings had the highest Lead concentration (10.83±1.27mg/kg). Fish had the highest Cadmium (4.20±1.01mg/kg) and Zinc (15.88±5.53mg/kg) concentrations. The order of organisms contamination is anadara>fish>bird droppings>crab>insect. The order of metal concentration is THC>Zn>Pb>Fe>Cd. In contrast, there was no significant difference between vertebrate and invertebrate organisms (F1, 148 = 0.08, P=0.78). Vertebrates have higher chemical composition (Cd, Pb and Zn) as compared to invertebrates (Fe and THC). Application/Improvement: Concentration of THC and heavy metals in most mangroves species were above the FAO/WHO standards. This implies that there is a horizontal THC and heavy metal contamination across trophic levels, which is detrimental to public health.
Keywords: Bioaccumulation; heavy metals; invertebrates; Niger delta; pollution; vertebrates
© 2020 Numbere. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Published By Indian Society for Education and Environment (iSee)
Subscribe now for latest articles and news.