

Indian Journal of Science and Technology
DOI: 10.17485/IJST/v15i24.1016
Year: 2022, Volume: 15, Issue: 24, Pages: 1187-1194
Original Article
Gourav Kumar Jain1*, Rajni Verma1, Arun Chougule1, Bharti Singh2
1Department of Radiological Physics, SMS Medical College & Hospital, Jaipur-302004, India
2Department of Chemistry, Indian Institute of Technology Delhi, New Delhi-110016, India
*Corresponding Author
Email: [email protected]
Received Date:11 May 2022, Accepted Date:28 May 2022, Published Date:01 July 2022
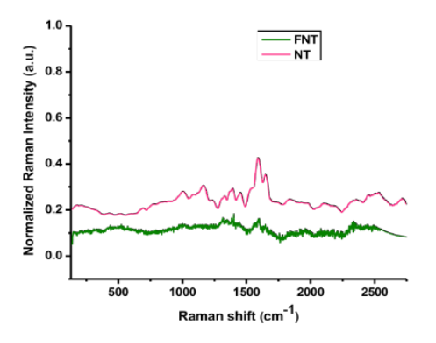
Novelty: Raman spectroscopy is extensively explored for the disease diagnostics in recent decade. There is limited literature available concerning the standardization of sample handling procedures in Raman spectroscopy research studies involving human subjects. In fact, the present study provided guidance for a harmonize data to conduct Raman spectroscopy research studies involving human subjects for better outcomes based on ethical principles. Objectives: Globally, multi-disciplinary research is conducted for better outcomes in the welfare of humankind. The present study was aimed to provide basic guidance of bioethical education for research students conducting research involving human subjects. Further, the present study was attempted to standardize sample handling procedure in Raman spectroscopy research involving human subjects for better outcomes in disease diagnosis. Materials and methods: We have provided bioethical recommendations based on fundamental ethics codes. The standardization of sample handling procedure was developed using human surgical samples of breast cancer patients. Findings: The researcher should justify the inclusion and exclusion criteria for biological samples to conduct the scientifically valid research study. Research studies involving biological samples shall develop research protocol for the preparation and handling of biological samples. The results of present study suggest that fresh clinically unprocessed tissue samples are superior to conduct research studies involving Raman spectroscopy for disease progression. In case the research is not concerned with positive net benefit for human participants with diagnosis of communicable diseases, the researcher shall exclude these patients from the research study. Informed consent, preferably in writing, has to be obtained from participants in a language in that participant comprehend. Conclusion: The present study described primary bioethical education and standardization techniques for research students to plan and conduct Raman spectroscopy research studies involving human biological materials.
Keywords: Raman spectroscopy; Bioethics; Cancer; Diagnosis; Spectrum
© 2022 Jain et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Published By Indian Society for Education and Environment (iSee)
Subscribe now for latest articles and news.