

Indian Journal of Science and Technology
Year: 2024, Volume: 17, Issue: 18, Pages: 1854-1859
Original Article
Jeeni Patel1, Dhara D Patel1*
1Department of Chemistry, Sankalchand Patel University, Visnagar, Gujarat, India
*Corresponding Author
Email: [email protected]
Received Date:27 February 2024, Accepted Date:11 April 2024, Published Date:30 April 2024
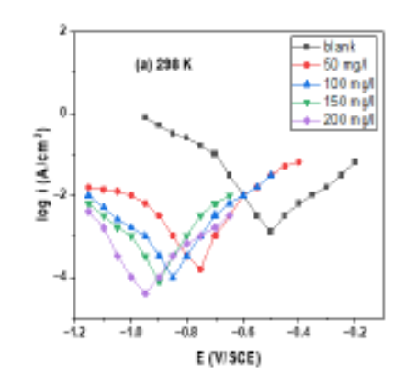
Objectives: To study the corrosion inhibition of Musa paradisiaca peel (MPP) extract for stainless steel (SS) 304. Method: The corrosion rate of stainless steel was studied by weight loss measurements in a 1 M hydrochloric acid solution at different concentrations of MPP inhibitor and at various temperatures, e.g., 298 K, 303 K, and 308 K. Scanning electron microscopy (SEM) was used to study the morphology of the films created on the stainless steel surface in various immersion media at various immersion times. Both potentiodynamic polarization (PDP) and electrochemical impedance spectroscopy (EIS) examined inhibition efficiency. Langmuir adsorption isotherm studied inhibitor surface adsorption SS 304. Findings: The corrosion rate and inhibition efficiency depend on the concentration of the inhibitor and the temperature. When there is an increase in the concentration of the inhibitor, inhibition efficiency increases, e.g., at 50 mg/l concentration, 77% inhibition efficiency, and at 200 mg/l concentration, 87% inhibition efficiency at 298 K. When temperature increases, inhibition efficiency decreases, e.g., at 200 mg/l concentration, 87% at 298 K, 85% at 303 K, and 83% at 308 K. Novelty: This article provides an eco-friendly corrosion inhibitor for stainless steel 304. Musa paradisiaca peel extract inhibitor was previously used for iron, mild steel, and carbon steel but was not used for stainless steel 304 in 1 M HCl.
Keywords: Corrosion, Stailess stell, Inhibitor, Acid
© 2024 Patel & Patel. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Published By Indian Society for Education and Environment (iSee)
Subscribe now for latest articles and news.