

Indian Journal of Science and Technology
DOI: 10.17485/IJST/v14i34.1424
Year: 2021, Volume: 14, Issue: 34, Pages: 2766-2772
Original Article
P Naresh Kumar Reddy1,2, Dadamiah P M D Shaik3 , D Nagamalleswari2 , K Thyagarajan4 , P Vishnu Prasanth2∗
1 Research Scholar, Department of Physics, JNTUA, Anantapuramu, 515002, India
2 Department of Physics, Sree Vidyanikethan Engineering College, A. Rangampet, 517102, India
3 Department of Physics, Lords Institute of Engineering and Technology, Hyderabad, 500091, India
4 Department of Physics, JNTUA College of Engineering, Pulivendula, 516390, India
∗ Corresponding author:
[email protected]
Received Date:01 August 2021, Accepted Date:05 October 2021, Published Date:25 October 2021
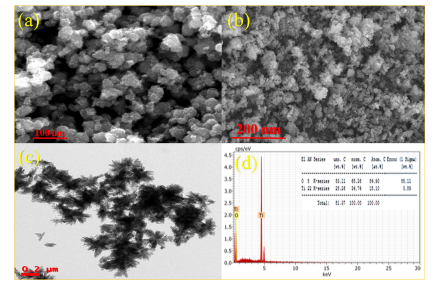
Objectives: Green synthesis of Titanium dioxide (TiO2) nanoparticles using Calotropis gigantea (CG) plant leaf extract. Methods: Environmental ecofriendly green approach is used to synthesize nanostructured TiO2 nanoparticles by using TiCl4 as a precursor and C. gigantea plant leaf extract as a catalyst. The secondary metabolites in the CG plant leaf extract help to transform the Ti4+ ions to TiO2 nanoparticles. The detailed structural properties are studied using X-ray diffraction (XRD), Field emission scanning electron microscopy (FESEM), and high-resolution transmission electron microscopy (HRTEM). The phase formation and chemical state of the prepared samples are examined by Raman and Energy Dispersive X-ray spectroscopy (EDX). The vibrational frequencies between the bonds of atoms are studied with Fourier Transform InfraRed spectroscopy (FTIR). The electrochemical properties of green synthesized nanoparticles using cyclic voltammetry (CV) technique in aqueous electrolyte. Findings: XRD data conform to the tetragonal structure of TiO2 in the rutile phase with P42/mnm space group and crystallite size is also found to be 9.84 nm. The SEM and TEM images show that the non-uniform spherical and flowerlike shape of grains with an average grains size of 100 nm. The specific capacitance of the sample is estimated to be 238 F g-1 at a scan rate of 1 mV s-1 with good reversibility. Novelty: The novelty of this research lies in the fabrication of the electrode material with TiO2 3D nanostructures for supercapacitor applications. This kind of morphology certainly enhances the surface area and leads to achieving better electrochemical performance.
Keywords: Titanium tetra chloride; TiO 2 nanoparticles; Green synthesis; Calotropis gigantea (CG) Plant; 3D nanostructure; specific capacitance
1) Reddy PNK, Shaik DPMD, Ganesh V, Nagamalleswari D, Thyagarajan K, Prasanth PV. High electrochemical activity of 3D flower like nanostructured TiO2 obtained by green synthesis. Applied Surface Science. 2021;561(150092). Available from: https://dx.doi.org/10.1016/j.apsusc.2021.150092.
2) Gonçalves RA, Toledo RP, Joshi N, Berengue OM. Green Synthesis and Applications of ZnO and TiO2 Nanostructures. Molecules. 2021;26(8):2236. Available from: https://dx.doi.org/10.3390/molecules26082236.
3) Yadav MS. Metal oxides nanostructure-based electrode materials for supercapacitor application. Journal of Nanoparticle Research. 2020;22(12):367. Available from: https://dx.doi.org/10.1007/s11051-020-05103-2.
4) Dubey RS. Temperature-dependent phase transformation of TiO2 nanoparticles synthesized by sol-gel method. Materials Letters. 2018;215:312–317. doi:10.1016/j.matlet.2017.12.120.
5) Wang D, Xie K, Wang Y, Cheng S. A Non-aqueous Hybrid Supercapacitor with Porous Anatase TiO2 Nanoparticles Anode and Activated Carbon Cathode. International Journal of Electrochemical Science. 2016;11:9776–9782. Available from: http://www.electrochemsci.org/papers/vol11/111209776.pdf.
6) Zikriya M, Nadaf YF, Bharathy PV, Renuka CG. Luminescent characterization of rare earth Dy3+ ion doped TiO2 prepared by simple chemical coprecipitation method. Journal of Rare Earths. 2019;37(1):24–31. Available from: https://dx.doi.org/10.1016/j.jre.2018.05.012.
7) Lee DH, Swain B, Shin D, Ahn NK, Park JR, Park KS. One-pot wet chemical synthesis of fluorine-containing TiO2 nanoparticles with enhanced photocatalytic activity. Materials Research Bulletin. 2019;109:227–232. Available from: https://dx.doi.org/10.1016/j.materresbull.2018.09.027.
8) Shaik DPMD, Kumar MVS, Reddy PNK, Hussain OM. High electrochemical performance of spinel Mn3O4 over Co3O4 nanocrystals. Journal of Molecular Structure. 2021;1241:130619. Available from: https://dx.doi.org/10.1016/j.molstruc.2021.130619.
9) Prakash NG, Dhananjaya M, Narayana AL, Shaik DP, Rosaiah P, Hussain OM. High Performance One Dimensional α-MoO3 Nanorods for Supercapacitor Applications. Ceramics International. 2018;44(8):9967–9975. Available from: https://dx.doi.org/10.1016/j.ceramint.2018.03.032.
10) Dhananjaya M, Prakash NG, Narayana AL, Hussain OM. Microstructural and supercapacitive properties of one-dimensional vanadium pentoxide nanowires synthesized by hydrothermal method. Applied Physics A. 2018;124(2):185. Available from: https://dx.doi.org/10.1007/s00339-017-1522-0.
11) Shaik DP, Pitcheri R, Qiu Y, Hussain OM. Hydrothermally synthesized porous Mn3O4 nanoparticles with enhanced electrochemical performance for supercapacitors. Ceramics International. 2019;45(2):2226–2233. Available from: https://dx.doi.org/10.1016/j.ceramint.2018.10.135.
12) Mathew S, Victório CP, S JSM, H BTB. Biosynthesis of silver nanoparticle using flowers of Calotropis gigantea (L.) W.T. Aiton and activity against pathogenic bacteria. Arabian Journal of Chemistry. 2020;13(12):9139–9144. Available from: https://dx.doi.org/10.1016/j.arabjc.2020.10.038.
13) Alexpandi R, Gopi CVVM, Durgadevi R, Kim HJ, Pandian SK, Ravi AV. Metal sensing-carbon dots loaded TiO2-nanocomposite for photocatalytic bacterial deactivation and application in aquaculture. Scientific Reports. 2020;10(1):12883. Available from: https://dx.doi.org/10.1038/s41598-020-69888- x.
14) Jin SE, Jin HE. Synthesis, Characterization, andThree-Dimensional Structure Generation of Zinc Oxide-Based Nanomedicine for Biomedical Applications. Pharmaceutics. 2019;11(11):575. Available from: https://dx.doi.org/10.3390/pharmaceutics11110575.
15) Saeed M, Usman M, Muneer M, Akram N, ul Haq A, Tariq M, et al. “Synthesis of ag-fe3o4 nanoparticles for degradation of methylene blue in aqueous medium”. Bulletin of the Chemical Society of Ethiopia. 2020;34(1):123–134. Available from: https://dx.doi.org/10.4314/bcse.v34i1.11.
16) Thakur PK, Verma V. A Review on Green Synthesis, Characterization and Anticancer Application of Metallic Nanoparticles. Applied Biochemistry and Biotechnology. 2021;10:1–22. Available from: https://dx.doi.org/10.1007/s12010-021-03598-6.
17) Yadav LSR, Manjunath K, Kavitha C, Nagaraju G. Investigation of Hydrogen generation and Antibacterial activity by Ionic liquid aided synthesis of TiO2 nanoparticles”. Journal of Science: Advanced Materials and Devices. 2018. Available from: 10.1016/j.jsamd.2018.03.002.
18) Abisharani JM, Devikala S, Kumar RD, Arthanareeswari M, Kamaraj P. Green synthesis of TiO2 Nanoparticles using Cucurbita pepo seeds extract. Materials Today: Proceedings. 2019;14(2):302–307. Available from: https://dx.doi.org/10.1016/j.matpr.2019.04.151.
19) Saeed M, Siddique M, Ibrahim M, Akram N, Usman M, Aleem MA, et al. Calotropis gigantea leaves assisted biosynthesis of ZnO and Ag@ZnO catalysts for degradation of rhodamine B dye in aqueous medium. Environmental Progress & Sustainable Energy. 2020;39(4):e13408. Available from: https://doi.org/10.1002/ep.13408.
20) Saeed M, Muneer M, Mumtaz N, Siddique M, Akram N, Hamayun M. Ag-Co 3 O 4 : Synthesis, characterization and evaluation of its photo-catalytic activity towards degradation of rhodamine B dye in aqueous medium. Chinese Journal of Chemical Engineering. 2018;26(6):1264–1269. Available from: https://dx.doi.org/10.1016/j.cjche.2018.02.024.
21) Venkatesan P, Remya N. Synthesis of TiO2 using Calotropis gigantea for Visible light Excitation and Degradation of pollutants. Toxic, and Radioactive Waste. 2021;25:4. Available from: https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000632.
22) Prashanth V, Priyanka K, Remya N. Solar photocatalytic degradation of metformin by TiO2 synthesized using Calotropis gigantea leaf extract. Water Science and Technology. 2021;83(5):1072–1084. Available from: https://dx.doi.org/10.2166/wst.2021.040.
23) Patil SP. Calotropis gigantea assisted green synthesis of nanomaterials and their applications: a review. Beni-Suef University Journal of Basic and Applied Sciences. 2020;9(1):14. Available from: https://doi.org/10.1186/s43088-020-0036-6.
24) Bekele ET, Gonfa BA, Sabir FK. Use of Different Natural Products to Control Growth of Titanium Oxide Nanoparticles in Green Solvent Emulsion, Characterization, and Their Photocatalytic Application. Bioinorganic Chemistry and Applications. 2021;2021(6626313):1–17. Available from: https: //dx.doi.org/10.1155/2021/6626313.
25) Bahria SS, Haruna Z, Hubadillahb SK, Norhayati W, Sallehc W, Rosmanc N, et al. Noor Hasliza Kamaruddina , Faiz Hafeez Azhara , Norsuhailizah Sazalia , Raja Adiba Raja Ahmada and Hatijah Basri. “Review on recent advance biosynthesis of TiO2 nanoparticles from plant-mediated materials: characterization, mechanism and application. IOP Conf Series: Materials Science and Engineering. 2021;p. 1142. doi:10.1088/1757-899X/1142/1/012005.
26) Nadeem M, Tungmunnithum D, Hano C, Abbasi BH, Hashmi SS, AhmadW, et al. The current trends in the green syntheses of titanium oxide nanoparticles and their applications. Green Chemistry Letters and Reviews. 2018;11(4):492–502. Available from: https://dx.doi.org/10.1080/17518253.2018.1538430.
27) Haldar KK, Banerjee B, Saha M, Ahmed I, Mete S, Patil RA, et al. Green Approach for the Fabrication of Au/ZnO Nanoflowers: A Catalytic Aspect. J Phys Chem C. 2021;125(12):6619–6631. Available from: https://doi.org/10.1021/acs.jpcc.0c10149.
28) Kantheti P, Alapati P. Green synthesis of TIO2 nanoparticles using Ocimum basilicum extract and its characterization. International Journal of Chemical Studies. 2018;6(4):670–674. Available from: http://krishi.icar.gov.in/jspui/handle/123456789/24553.
29) Gharge VG, Yadav A. ”Study of Methanolic Extract of Leaves Calotropis gigantea (L.) As an Anti-Solar”. Advances in Complementary & Alternative Medicine. 2018;1(4). Available from: 10.31031/ACAM.2018.01.000518.
30) Singh R, Dutta S. Synthesis and characterization of solar photoactive TiO 2 nanoparticles with enhanced structural and optical properties. Advanced Powder Technology. 2018;29(2):211–219. Available from: https://dx.doi.org/10.1016/j.apt.2017.11.005.
31) Nwankwo U, Bucher R, Ekwealor ABC, Khamlich S, Maaza M, Ezema FI. Synthesis and characterizations of rutile-TiO2 nanoparticles derived from chitin for potential photocatalytic applications. Vacuum. 2019;161:49–54. Available from: https://dx.doi.org/10.1016/j.vacuum.2018.12.011.
32) Ekoi EJ, Gowen A, Dorrepaal R, Dowling DP. Characterisation of titanium oxide layers using Raman spectroscopy and optical profilometry: Influence of oxide properties. Results in Physics. 2019;12:1574–1585. Available from: https://dx.doi.org/10.1016/j.rinp.2019.01.054.
33) Sarkar A, Khan GG. The formation and detection techniques of oxygen vacancies in titanium oxide-based nanostructures. Nanoscale. 2019;11(8):3414– 3444. Available from: https://dx.doi.org/10.1039/c8nr09666j.
34) Ojha DP, Poudel MB, Kim HJ. Investigation of electrochemical performance of a high surface area mesoporous Mn doped TiO2 nanoparticle for a supercapacitor. Materials Letters. 2020;264:127363. Available from: https://dx.doi.org/10.1016/j.matlet.2020.127363.
35) Parveen N, Cho MH. Self-Assembled 3D Flower-Like Nickel Hydroxide Nanostructures and Their Supercapacitor Applications. Scientific Reports. 2016;6(1):27318. Available from: https://dx.doi.org/10.1038/srep27318.
36) Wang D. A Non-aqueous Hybrid Supercapacitor with Porous Anatase TiO2 Nanoparticles Anode and Activated Carbon Cathode. International Journal of Electrochemical Science. 2016;11:9776–9782. doi:10.20964/2016.12.15.
37) Agharezaei P, Abdizadeh H, Golobostanfard MR. Flexible supercapacitor electrodes based on TiO2/rGO/TiO2 sandwich type hybrids. Ceramics International. 2018;44(4):4132–4141. Available from: https://dx.doi.org/10.1016/j.ceramint.2017.11.214.
38) Qin Y, Zhang J, Wang Y, Shu X, Yu C, Cui J, et al. Supercapacitive performance of electrochemically doped TiO2 nanotube arrays decorated with Cu2O nanoparticles. RSC Advances. 2016;6(53):47669–47675. Available from: https://dx.doi.org/10.1039/c6ra08891k.
39) Zhao Y, Xu L, Huang S, Bao J, Qiu J, Lian J, et al. Facile preparation of TiO2/C3N4 hybrid materials with enhanced capacitive properties for high performance supercapacitors. Journal of Alloys and Compounds. 2017;702:178–185. Available from: https://dx.doi.org/10.1016/j.jallcom.2017.01.125.
40) Kim H, Cho MY, Kim MH, Park KY, Gwon H, Lee Y, et al. A Novel High-Energy Hybrid Supercapacitor with an Anatase TiO2-Reduced Graphene Oxide Anode and an Activated Carbon Cathode. Advanced Energy Materials. 2013;3(11):1500–1506. Available from: https://dx.doi.org/10.1002/aenm. 201300467.
41) Anandhia P, Kumar V, Harikrishnan S. Enhanced Capacitive Characteristics of TiO2 Nanoflakes Based Electrode Material For Supercapacitor”. Journal of Electrical Engineering. 2017;1:252–258.
42) Reddy PNK, Shaik DP, Ganesh V, Nagamalleswari D,Thyagarajan K, Prasanth PV. Structural, optical and electrochemical properties of TiO2 nanoparticles synthesized using medicinal plant leaf extract. Ceramics International. 2019;45(13):16251–16260. Available from: https://dx.doi.org/10.1016/j.ceramint. 2019.05.147.
© 2021 Naresh Kumar Reddy et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Published By Indian Society for Education and Environment (iSee)
Subscribe now for latest articles and news.