

Indian Journal of Science and Technology
DOI: 10.17485/IJST/v16i46.2995
Year: 2023, Volume: 16, Issue: 46, Pages: 4436-4444
Original Article
Rosita Ruth Carlota1, Anjali Nayak2*, Yashwanth V Reddy1, K R Prathibha1, T Nirma1, Daphisha Marbaniang1
1Department of Quality Assurance, Krupanidhi College of Pharmacy, Bangalore, 560 035, Karnataka, India
2Department of Pharmaceutical Chemistry, Krupanidhi College of Pharmacy, Bangalore, 560035, Karnataka, India
*Corresponding Author
Email: [email protected]
Received Date:23 November 2023, Accepted Date:08 December 2023, Published Date:20 December 2023
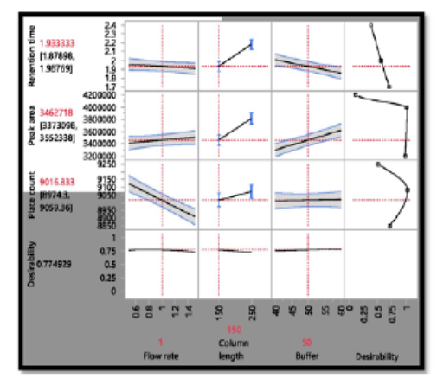
Objectives: This study aimed at the methodical design and validation of a Reversed-Phase High-Performance Liquid Chromatographic method (RP-HPLC) for the estimation of Levosulpiride in bulk form using analytical quality by design approach. Methods: Using custom design, the Critical Method Parameters were methodically optimized. Peak area, retention time, and peak tailing were assessed for flow rate, buffer, and column length as the influencing elements for Critical Analytical Attributes and using an Agilent C8 column in isocratic elution mode with mobile phase NaH2PO4 buffer and methanol (50:50% v/v) and flow rate at 1ml/min. Findings: Chromatographic separation was accomplished on the Agilent C8 (150×4.6 mm, 5m) column. The optimized and predicted data from JMP PRO 14 software consisted of mobile phase NaH2PO4(50%): Methanol (50%), pumped at a flow rate of 1 ml/min which gave the higher desirability function of 77%. LOD and LOQ values were 0.06 g/ml and 0.20 g/ml respectively and models were found to be significant (p < 0.05). The validation parameter findings were within the permitted range. Forcefully testing the drug's stability under various stress situations revealed considerable degradation in the presence of heat. Novelty: The decrease in the retention and run time shows that the method is simple, accurate, precise and economical for the estimation of Levosulpiride that can be adopted in regular quality control tests in industries.
Keywords: Analytical quality by design, Custom design, Desirability function, Levosulpiride, Reversed Phase Liquid Chromatography
© 2023 Carlota et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Published By Indian Society for Education and Environment (iSee)
Subscribe now for latest articles and news.