

Indian Journal of Science and Technology
Year: 2022, Volume: 15, Issue: 23, Pages: 1159-1165
Original Article
Jayshree Rathore1*, Arya Rakesh Kumar1, Pratibha Sharma1, Mohan Lal1
1Photochemical laboratory, Department of Chemistry, Faculty of Science, Jai Narain Vyas University, Jodhpur (Rajasthan) 342001, India
*Corresponding Author
Email: [email protected]
Received Date:01 March 2022, Accepted Date:09 May 2022, Published Date:29 June 2022
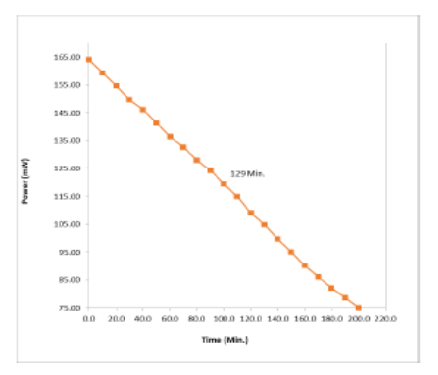
Objective: The present study is focusing on the role of surfactant in photogalvanic cells and how photons from sunlight can be used as a driving force for energy solar energy conversion and storage. Methods: An H shaped cell was designed for study of electrical output in solar transformations. Electrical circuit was proposed by using of dye, reductant, surfactant, NaOH, double distilled water (DDW), multi-meter, calomel electrode, 250 k roistered, saturated calomel electrode, platinum electrode, carbon pot, resistance key, digital pH meter, microammeter, and 200 W tungsten bulb. A detailed reaction mechanism for the proposed photogalvanic cell (PG Cell) for generating photocurrent and photocurrent has been studied. PG Cells were studied for the solar energy transformation system. Findings: PG Cells were studied by using different parameters via photocurrent, photopotential, conversion efficiency, fill factor and cell performance. The above values are as follows: 388.0 □A, 1141.0 mV, 0.7995%, 0.5389 and 129.0 minutes. Electrical output of the cell has also been observed for tartrazine, D-Fructose and lauryl glucoside systems. Potential at power point, Potential at open circuit, power point of cell (pp) and current at short circuit were also studied. The obtained values are as follows: 1133 mV, 1523 mV, 435.321 and 544mA. Novelty: The photogalvanic is an emerging field of research for conversion and storage of solar energy. This study employs lauryl glucoside-tartrazine-D-fructose system for better electrical output which is also an eco-friendly natural dye system. The observed results are better in cell performance (t1=2) and reduction in cost (INR 9531.38 / USD 125) of the photogalvanic cell for its commercial viability.
Keywords: Photogalvanic cells; solar energy conversion; storage; lauryl glucoside; tartrazine
© 2022 Rathore et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Published By Indian Society for Education and Environment (iSee)
Subscribe now for latest articles and news.