

Indian Journal of Science and Technology
DOI: 10.17485/IJST/v13i34.1575
Year: 2020, Volume: 13, Issue: 34, Pages: 3510-3514
Original Article
Dalal Alarabi1, Abdulkarim Abdulrahman2, Salman Alali3, Omar Yaghi1,Manaf Alqahtani3,4*
1Bahrain National Taskforce to Combat COVID-19, Bahrain
2Bahrain National Taskforce to Combat COVID-19, Mohammed bin Khalifa Cardiac Centre,Bahrain
3Bahrain National Taskforce to Combat COVID-19, Bahrain Defense Force Hospital, Bahrain
4Consultant Infectious disease and clinical microbiologist, Bahrain Defence Force hospital,
Bahrain. Tel.: +973 3976 6000
*Corresponding Author
Tel: +973 3976 6000
Email: [email protected]
Received Date:02 September 2020, Accepted Date:09 September 2020, Published Date:15 September 2020
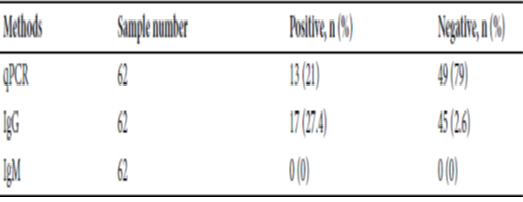
Objectives: This study evaluated VivaDiag IgM/IgG Rapid Test (VDtest) for covid-19 screening, during disease progression and following recovery. Methods: Prospectively, 969 patients RT-PCR positive for SARS-CoV-2 virus were compared to VDtest in 166 individuals upon airport arrival; 62 active inpatient COVID-19 cases ranging 2-23 days; 741 recovered COVID-19 patients from diagnosis date (median 24-days). Findings: Screening; VDtest assay sensitivity 7.6% (95% CI 2.8–15.8%), specificity 94.3% (95% CI 87.1–98.1%). Active disease patients, positive IgG rate 27.4% and IgM positivity 0 of 62 patients. Recovery phase patients: positive rates of IgM and IgG were 0.7% and 1.2%, respectively, within 14-days of diagnosis date, increasing to 25.9% and 43.4%, respectively 14-days after diagnosis. Novelty: VDtest kit showed poor sensitivity and identification of COVID-19 infection for screening, moreover,need for larger sample study to confirm our findings.
Keywords: COVID19; SARSCoV2; RTPCR; serological testing; rapid test
© 2020 Alarabi et al.This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Published By Indian Society for Education and Environment (iSee).
Subscribe now for latest articles and news.