

Indian Journal of Science and Technology
Year: 2020, Volume: 13, Issue: 28, Pages: 2892-2901
Original Article
Alaa E shams1, Amal F Soliman2, Ahmed Ashour2*, Amani M Marzouk1,2,Saleh H Elsharkawy2
1Department of pharmacognosy, Faculty of pharmacy, Delta University for Science and Technology, Gamasa City, Coastal international road, Egypt
2Department of Pharmacognosy, Faculty of Pharmacy, Mansoura University, Mansoura,35516, Egypt. Tel.: 0200101028921
*Corresponding Author
Tel: 0200101028921
Email: [email protected]
Received Date:13 June 2020, Accepted Date:26 July 2020, Published Date:07 August 2020
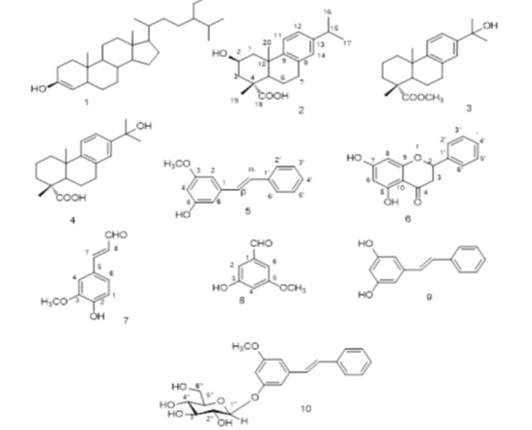
Objectives: This study aims to isolate and identify valuable chemicals and pharmaceuticals from the waste of wood (Pinus sylvestris L.) industry and to determine their biological value. Methods: Chromatographic technique was used to isolate compounds from the waste. The chemical identity of the isolated compounds were evidenced by different spectroscopic analyses as well as comparative studies of reported data. The microbial transformed product of pinosylvin mono methyl ether was quantitatively determined using fluorimetric method. All the isolated compounds were tested for their antitoxoplasmosis activity (in vitro) against Toxoplasma gondii. Findings/ Novelty: Nine compounds were isolated and identified including three diterpenes : (1b -hydroxy dehydroabietic acid, 15-hydroxy dehydroabietic acid and Methyl-15-hydroxy dehydroabiet-18-ate) ; two aromatic aldehydes (coniferaldhyde and 3-hydroxy, 5-methoxy benzaldhyde) ; two stilbene derivatives; (Pinosylvin and Pinosylvin mono methyl ether) ; a flavan: (Pinocembrin) and a phytosterol (b -sitosterol).For the first time, the fluorimetric method was used for quantitative determination of the novel metabolite produced, E-Pinosylvin mono methyl ether 5-O-b -D-glucopyranoside, from the microbial biotransformation technique. All isolated compounds showed variable antitoxopasmosis activity, carried out for the first time, against T. gondii according to their % mortality and recorded EC50.
Keywords: Pinus sylvestris L.; saw dust; antitoxoplasmosis; fluorimetry; microbial transformation
© 2020 shams et al..This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Published By Indian Society for Education and Environment (iSee).
Subscribe now for latest articles and news.