

Indian Journal of Science and Technology
DOI: 10.17485/IJST/v15i43.1264
Year: 2022, Volume: 15, Issue: 43, Pages: 2282-2289
Original Article
Subitha Mani1, Vasanthi Nachiappan1*
1Bio-membrane Lab, Department of Biochemistry, School of Life Sciences, Bharathidasan University, Tiruchirappalli, 620024, Tamilnadu, India
*Corresponding Author
Email:
Received Date:15 June 2022, Accepted Date:04 October 2022, Published Date:16 November 2022
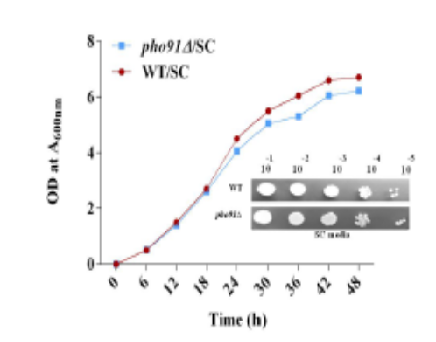
Objective: The current study tries to elucidate the impact of low-affinity Pi transporter Pho91 (using pho91Δ cells) on lipid metabolism and cellular organelles morphology (mitochondria and vacuole) in yeast Saccharomyces cerevisiae. Methods: The lipid profile was performed using thin layer chromatography (TLC), and the membrane defect was determined using DiOC6 staining, lipid droplets (LDs) were observed by Nile red staining, mitochondrial morphology was observed using aconitase 1 GFP, vacuolar morphology was studied by FM4-64 staining and were performed with the aid of laser scanning fluorescent microscope. Findings: Pho91 is a low-affinity phosphate (Pi) transporter in the vacuoles that functions under Pi-rich conditions, but its role in lipid metabolism is largely unknown. In this study, we used defined synthetic complete media (SC). The deletion of Pho91 depicted a moderate growth defect but increased the major phospholipids PC, PE, and PI. Alterations in the membrane phospholipids resulted in defective membrane morphology. In pho91 mutant (pho91Δ), the neutral lipids TAG and SE were increased and stored as LDs. The LD numbers were increased in pho91Δ cells than in WT cells. Altered phospholipids also defective mitochondrial morphology and enlarged vacuoles in pho91 deletion. Novelty: In the absence of low-affinity Pi transporter pho91, it increases phospholipid, neutral lipid levels, LD numbers, and impacted mitochondria along with vacuolar structures.
Keywords: Phosphate transporter; Low affinity; Phospholipids; Neutral lipids; Lipid droplets
© 2022 Mani & Nachiappan. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Published By Indian Society for Education and Environment (iSee)
Subscribe now for latest articles and news.