

Indian Journal of Science and Technology
Year: 2021, Volume: 14, Issue: 31, Pages: 2550-2556
Original Article
Muthumani Mamudha1*, Anbu Jeba Sunilson2
1Research scholar, Department of siddha medicine, Tamil university, Thanjavur, India
2Associate professor, Department of siddha medicine, Tamil university, Thanjavur, India
*Corresponding Author
Email: [email protected]
Received Date:27 May 2021, Accepted Date:16 August 2021, Published Date:22 September 2021
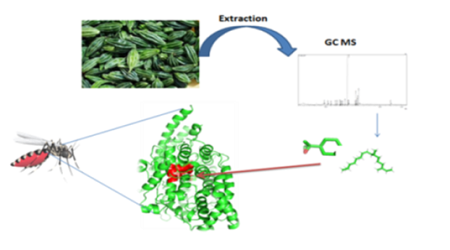
Objectives: This study aimed to identify phytochemicals from Momordica tuberosa (M. tuberosa) leaves and it’s in silico larvicidal potential compounds against Anopheles. Further, to analyze their inhibitory mechanisms against the Anopheles angiotensin-converting enzyme (ACE). In view of the above fact, we made an attempt to discover potential and novel natural mosquito larvicidal compounds with least impact on human health. Method: The GC-MS analysis confirmed the phytoconstituents from the methanol extracts of M. tuberosa through GC-MS and selected compounds were submitted to docking studies on Anopheles angiotensin-converting enzyme as possible targets. Findings: GC-MS analysis of the extract showed the presence of 20 compounds with Limonene dioxide, Neophytadiene and Palmitic acid as main constituents. Three Selected compounds (Limonene dioxide, Neophytadiene and palmitic acid) specifically binds the binding site of an angiotensin-converting enzyme (ACE). The binding energy Limonene dioxide (- 6 kcal/mol), Neophytadiene (- 6.5 kcal/mol), Palmitic acid (- 5.7 kcal/mol) and interaction were analyzed. The present study displays better docking binding energy in the selected compounds. The results further substantiate that the 3 compounds of the M. tuberose found to be model candidates for the design and development of potential larvicidal. Novelty: The study helps to pave the first way to develop the promising larvicidal compound from plants. This result visibly suggests that compounds (Limonene dioxide, Neophytadiene and Palmitic acid) from M. tuberosa could act as potential larvicidal agents – to control Anopheles.
Keywords: Phytochemical; Molecular docking; anangiotensinconverting enzyme (ACE); larvicidal; Chromatography
© 2021 Mamudha & Sunilson. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Published By Indian Society for Education and Environment (iSee)
Subscribe now for latest articles and news.