

Indian Journal of Science and Technology
Year: 2022, Volume: 15, Issue: 30, Pages: 1504-1516
Original Article
Vishakha Vishwanath Doke1*, Nilesh M Khutle2, Maya Sharma3, Khemchand Gupta4
1Research Scholar (Ph.D.), Faculty of Pharmacy, Pacific Academy of Higher Education and Research University, Rajasthan, Udaipur, India
2Assistant Professor (Pharmaceutics), Dr. L. H. Hiranandani College of Pharmacy, Ulhasnagar, Maharashtra, India
3Associate Professor, Pacific college of Pharmacy, Pacific Academy of Higher Education and Research University, Udaipur, Rajasthan, India
4Professor and Principle, Venkateshwar Institute of pharmacy, Sai Tirupati University, Udaipur, Rajasthan, India
*Corresponding Author
Email: [email protected]
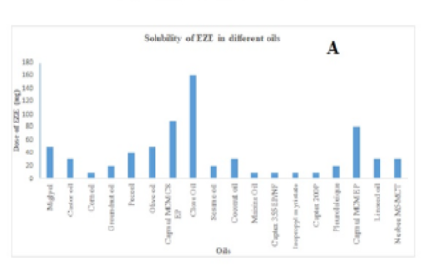
Objectives: To enhance solubility, dissolution, and permeability of poorly water-soluble drug Ezetimibe (EZE) using a self-nano emulsifying drug delivery system (SNEDDS). Methods: Initially, the solubility of the EZE was determined in various oils and buffers. Surfactants and co-surfactants were screened based on the solubility of the drug in oil as per the emulsification efficacy test. Liquid SNEDDS was developed and characterized. Solid SNEDDS was developed and characterized using optimized liquid SNEDDS followed by the development of EZE-loaded Tablet SNEDDS. Finding: Liquid SNEDDS (LSNEDDS) was formulated using Capmul MCM C8 EP, Cremophore RH 40, and Labrafil M 2125 CS as oil, surfactant, and co-surfactant respectively. Optimized L-SNEDDS formulation was found to be efficient with an average %T of 99.5%, drug content of 98.43%, flask inversion 0 numbers, and the average particle size of 36.7 nm, zeta potential of -57.5 mV, Polydispersibility index in of 0.119. Also, the in vitro release profile of drug from L-SNEDDS encapsulated in hard gelatin capsules was evaluated in different dissolution media viz. simulated gastric fluid and simulated intestinal fluid. For the drug, more than 95% cumulative release was observed within 30 min exclusive of the pH of the medium. The L-SNEDDS were adsorbed on a solid support and then mixed with tablet blends and compressed into tablets. Further, no adverse changes in globule size, shape, the zeta potential of SNEDDS, and dissolution profile were apparent on conversion to solid powder form and tablet form. Novelty: The developed liquid SNEDDS form of EZE showed enhanced solubility, dissolution, and permeability in comparison to pure drugs. Conversion of L-SNEDDS to PSNEDDS would be a novel approach to overcome the limitations associatedwith liquid dosage forms.
Keywords: Ezetimibe (EZE); Selfnano emulsifying drug delivery system (SNEDDS); liquid SNEDDS (L SNEDDS); powder SNEDDS (PSNEDDS); Tablet SNEDDS (TSNEDDS)
© 2022 Doke et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Published By Indian Society for Education and Environment (iSee)
Subscribe now for latest articles and news.